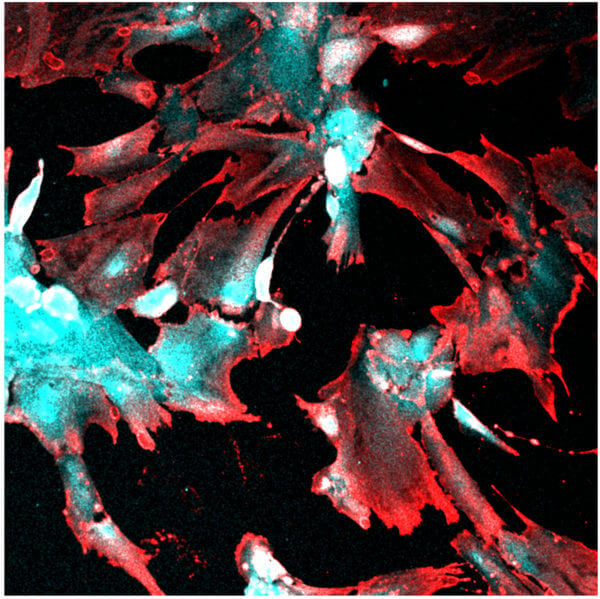
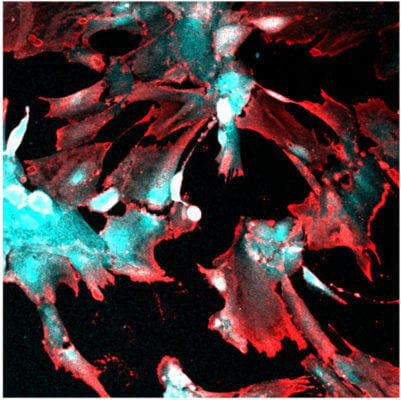
The virus that causes COVID-19, SARS-CoV-2, can directly infect a specialized type of kidney cell. The discovery helps explain why acute kidney injury is one of the main complications observed in patients with severe COVID-19, according to biomedical engineers and virologists at Duke University.
The research appeared online April 20 in the journal Frontiers in Cell and Developmental Biology.
When COVID-19 began spreading across the globe in early 2020, physicians knew that the virus primarily infected cells in the respiratory tract. But as the case numbers began to grow, physicians were surprised to see that many patients –– especially those with severe COVID-19 –– were also developing injuries to their kidneys.
The issue came to Samira Musah’s attention when she attended a virtual symposium in the spring of 2020. Musah, an assistant professor of biomedical engineering and medicine at Duke, listened as physicians presented research that described how patients who had never experienced any kidney-related issues were developing kidney disease after getting sick with COVID-19.
“It was shocking to hear doctors describe how patients who were healthy suddenly developed kidney injury and needed to go on dialysis after contracting SARS-CoV-2,” said Musah. “It was clear that the virus was doing something to the kidneys, but it was so early in the pandemic that nobody was sure what was going on.”
In previous work, Musah and her team showed that they could guide human induced pluripotent stem cells to develop and mature into functional podocytes, which is a specific type of kidney cell that helps control the removal of toxins and waste from the blood. Musah and Titilola Kalejaiye, a postdoctoral fellow in the lab, wanted to see if they could use this model to determine how and why SARS-CoV-2 was capable of damaging kidney cells.
As a proof of concept, Kalejaiye initially worked with a pseudovirus version of SARS-CoV-2. These psuedoviruses are developed to mimic characteristics of specific viruses but are incapable of producing replication-competent viral particles, making them safe to use for broad research. After introducing the pseudovirus into their podocyte cell model, Kalejaiye discovered that the spike protein of the virus could directly bind to numerous receptors on the surface of podocytes.
“We found that the virus was especially adept at binding to two key receptors on the surface of the podocytes, and these receptors are abundant in these kidney cells,” explained Kalejaiye, who is also the first author of the paper. “There was a strong uptake of the virus initially, and we also found that when you increased the dose of the virus, the uptake would increase even further. The virus seemed to have a strong affinity for these kidney cells.”
To test their podocyte model with the real SARS-CoV-2 virus, Musah and Kalejaiye teamed up with Maria Blasi, an assistant professor of medicine at Duke and a researcher in the Duke Human Vaccine Institute. Before the pandemic, Blasi was exploring how viruses, including HIV, infect and damage another subset of kidney cells called renal tubular epithelial cells.
“It was a stroke of luck that we crossed paths at the faculty meeting we both attended,” said Blasi. “Samira was looking for someone with experience handling live viruses, and I was looking for a model of the podocytes that Samira can make, so we decided to kill two birds with one stone.”
Just like with the pseudovirus, the team observed that the live version of the virus had a strong affinity for podocytes. Once the virus infected the cells, it damaged the podocytes, causing their long, finger-like structures, which help filter blood, to retract and shrivel. If the injuries to the cells were too severe, the podocytes would die.
“Beyond the structural damage, we saw that the virus could hijack the machinery of the podocytes to produce additional viral particles that could spread to infect additional cells,” Blasi said.
Now the team hopes to expand their work to study how the different variants of SARS-CoV-2 behave in kidney cells. As variants of the virus have emerged, kidney injuries are occurring less frequently. This has made the team question how the new variants are changing and if they are becoming less capable of infecting kidney cells.
“I think it’s remarkable that we went from being home and hearing the initial reports from physicians to forming this collaboration virtually and having these results on such a short timeline,” said Musah. “We had the right people and the right tools at the right time. It’s been one of the most successful collaborations of my relatively young lab, and I’m looking forward to continuing this work.”
This work was supported by a Whitehead Scholarship in Biomedical Research, a Chair’s Research Award from the Department of Medicine at Duke University, a Duke MEDx Pilot Grant on Biomechanics in Injury or Injury Repair, a Burroughs Welcome Fund PDEP Career Transition Ad Hoc Award, a Genentech Research Award, and a George M. O’Brien Kidney Center Pilot Grant (P30 DK081943) awarded to Musah. Blasi was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant number R01DK130381. Work with live SARS-CoV-2 isolate was performed under Biosafety Level-3 (BSL3) in the Duke Regional Biocontainment Laboratory (RBL), which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607, G20-AI167200).
CITATION: “SARS-CoV-2 Employ BSG/CD147 and ACE2 Receptors to Directly Infect Human Induced Pluripotent Stem Cell-Derived Kidney Podocytes,” Titilola Dalejaiye, Rohan Bhattacharya, Morgan Burt, Tatianna Travieso, Arinze Okafor, Xingrui Mou, Maria Blasi & Samira Musah. Frontiers in Cell and Developmental Biology, April 20, 2022. DOI: 10.3389/fcell.2022.855340
from ScienceBlog.com https://ift.tt/oWz1nfU
No comments:
Post a Comment